Regulatory submissions Courses
Explore instructor-led and hybrid programs aligned to practical Regulatory submissions use cases across industries.
Available Regulatory submissions Courses
Showing 1-5 of 5 courses

Biotechnology and Pharmaceutical Development
Advanced Regulatory Strategy for Diagnostics Training Course provides the next-level expertise needed to transition from reactive compliance to a proactive, globally-aligned regulatory function that minimizes risk, optimizes product lifecycles, and accelerates the delivery of next-generation diagnostic solutions to patients worldwide
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
Advanced Regulatory Strategy for Diagnostics Training Course provides the next-level expertise needed to transition from reactive compliance to a proactive, globally-aligned regulatory function that minimizes risk, optimizes product lifecycles, and accelerates the delivery of next-generation diagnostic solutions to patients worldwide
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore

Biotechnology and Pharmaceutical Development
Advanced Regulatory Strategy for Orphan Drugs Training Course is designed to move beyond the basics of designation, focusing instead on lifecycle management, global harmonization (ICH), and strategic commercialization planning from pre-clinical through post-approval.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
Advanced Regulatory Strategy for Orphan Drugs Training Course is designed to move beyond the basics of designation, focusing instead on lifecycle management, global harmonization (ICH), and strategic commercialization planning from pre-clinical through post-approval.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore

Biotechnology and Pharmaceutical Development
Medical Device Regulatory Affairs (ISO 13485) Training Course is essential for ensuring product safety and efficacy throughout the entire lifecycle, from design and development to post-market surveillance
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
Medical Device Regulatory Affairs (ISO 13485) Training Course is essential for ensuring product safety and efficacy throughout the entire lifecycle, from design and development to post-market surveillance
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore

Food processing and Technology
Regulatory Pathway for New Food Additives Training Course provides comprehensive guidance on understanding the regulatory framework, compliance requirements, and approval processes necessary for launching new food additives successfully.
5 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
Regulatory Pathway for New Food Additives Training Course provides comprehensive guidance on understanding the regulatory framework, compliance requirements, and approval processes necessary for launching new food additives successfully.
5 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
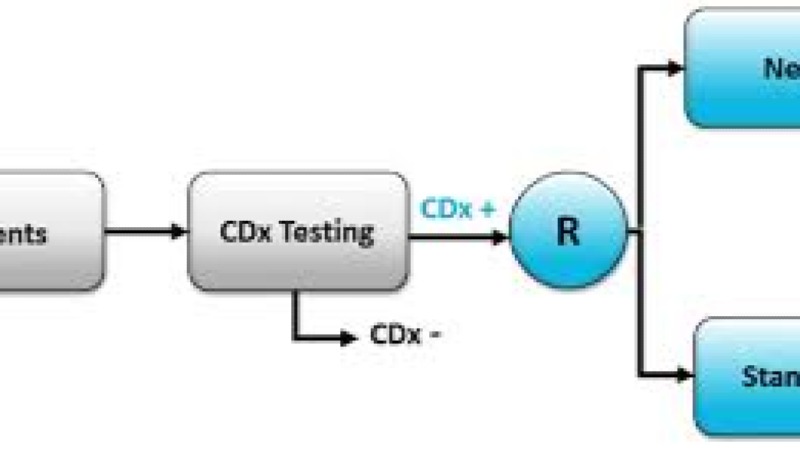
Biotechnology and Pharmaceutical Development
Regulatory Strategy for Companion Diagnostics Training Course is designed to equip professionals with the knowledge and strategic acumen needed to navigate this complex landscape, from initial biomarker discovery and co-development to market access and post-market surveillance.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
Regulatory Strategy for Companion Diagnostics Training Course is designed to equip professionals with the knowledge and strategic acumen needed to navigate this complex landscape, from initial biomarker discovery and co-development to market access and post-market surveillance.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore