Cip/sip systems Courses
Explore instructor-led and hybrid programs aligned to practical Cip/sip systems use cases across industries.
Available Cip/sip systems Courses
Showing 1-3 of 3 courses

Biotechnology and Pharmaceutical Development
Advanced Biofilm Control, Bioprocessing Contamination, cGMP Microbial Control, Sterility Assurance Training, Aseptic Processing, CIP SIP Validation, Quorum Sensing Inhibition, Anti-Biofilm Strategies, Process Analytical Technology (PAT), Extracellular Polymeric Substance (EPS), Biofilm Detection, Biomanufacturing Quality, Root Cause Analysis (RCA), Disinfectant Rotation, Biofilm-Resistant Materials, Single-Use Systems (SUS) Sterility, FDA EMA Compliance, Enzymatic Biofilm Removal, Bacteriophage Therapy, Process Hygiene
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
Advanced Biofilm Control, Bioprocessing Contamination, cGMP Microbial Control, Sterility Assurance Training, Aseptic Processing, CIP SIP Validation, Quorum Sensing Inhibition, Anti-Biofilm Strategies, Process Analytical Technology (PAT), Extracellular Polymeric Substance (EPS), Biofilm Detection, Biomanufacturing Quality, Root Cause Analysis (RCA), Disinfectant Rotation, Biofilm-Resistant Materials, Single-Use Systems (SUS) Sterility, FDA EMA Compliance, Enzymatic Biofilm Removal, Bacteriophage Therapy, Process Hygiene
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore

Biotechnology and Pharmaceutical Development
Advanced Cleaning Validation for Multi-Product Facilities Training Course is specifically designed to address the complex challenges of cleaning validation in modern multi-product pharmaceutical and biotechnology facilities.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
Advanced Cleaning Validation for Multi-Product Facilities Training Course is specifically designed to address the complex challenges of cleaning validation in modern multi-product pharmaceutical and biotechnology facilities.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
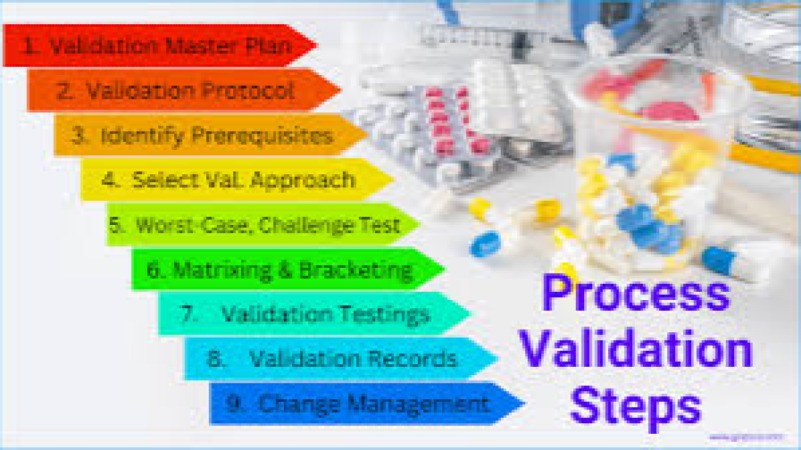
Biotechnology and Pharmaceutical Development
Advanced Process Validation & Cleaning Validation Training Course is meticulously designed to move beyond foundational GMP and GxP concepts, focusing on the modern, Science and Risk-Based Approach mandated by global regulatory bodies.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
Advanced Process Validation & Cleaning Validation Training Course is meticulously designed to move beyond foundational GMP and GxP concepts, focusing on the modern, Science and Risk-Based Approach mandated by global regulatory bodies.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore